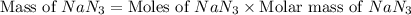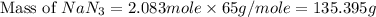Chemistry, 31.07.2019 17:10 Killion2022
Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. this causes sodium azide (nan3) to decompose explosively according to the following reaction: 2nan3 1s2 h 2na 1s2 1 3n2 1g2 what mass of nan3(s) must be reacted to inflate an air bag to 70.0 l at stp?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1

Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
Air bags are activated when a severe impact causes a steel ball to compress a spring and electricall...
Questions in other subjects:


History, 27.05.2020 23:02

Mathematics, 27.05.2020 23:02



Mathematics, 27.05.2020 23:02

Mathematics, 27.05.2020 23:02


Mathematics, 27.05.2020 23:02

Social Studies, 27.05.2020 23:02

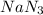 reacted will be, 135.395 grams.
reacted will be, 135.395 grams.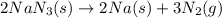
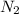 gas.
gas.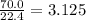 mole of
mole of 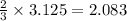 moles of
moles of