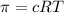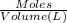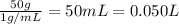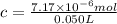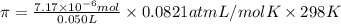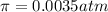Chemistry, 30.07.2019 23:30 makwoods417ow2txa
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this protein is dissolved in 50 g of water at 298 k. calculate the vapor pressure lowering, the depression in freezing point, the elevation of boiling point, and the osmotic pressure of this solution. the vapor pressure of pure water at 298 k is 23. 76 mmhg.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this...
Questions in other subjects:


Mathematics, 30.03.2020 23:37


Biology, 30.03.2020 23:37


Mathematics, 30.03.2020 23:37




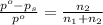
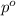 = Vapor Pressure of the pure solvent
= Vapor Pressure of the pure solvent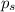 = Vapor Pressure of the solution
= Vapor Pressure of the solution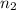 moles of solute
moles of solute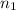 = moles of solvent
= moles of solvent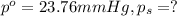
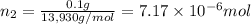
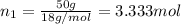
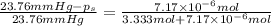
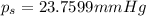
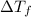 is given by:
is given by: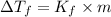
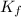 = molal depression constant of solvent
= molal depression constant of solvent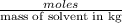
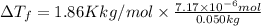
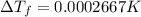
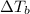 is given by:
is given by: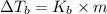
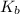 = molal elevation constant of solvent
= molal elevation constant of solvent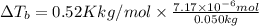
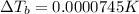
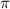 is given as:
is given as: