Chemistry, 30.07.2019 23:20 SydneyFrank
How many moles of carbon dioxide are produced when 5.12 mol of propane gas is burned in excess oxygen? c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(l)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 23.06.2019 07:30, jessicawelch25
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2

Chemistry, 23.06.2019 11:30, starfox5454
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2

Chemistry, 23.06.2019 11:30, nickolasbradyp0hvwl
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
How many moles of carbon dioxide are produced when 5.12 mol of propane gas is burned in excess oxyge...
Questions in other subjects:

Mathematics, 28.09.2020 14:01



Spanish, 28.09.2020 14:01

Biology, 28.09.2020 14:01



Social Studies, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

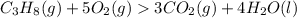
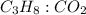 is 1 : 3
is 1 : 3
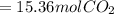 is produced.
is produced.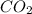 is
is