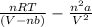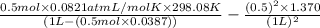Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1


Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2
You know the right answer?
Calculate the pressure exerted by 0.5000 mol of n2 in a 1.-l container at 25.08c a. using the ideal...
Questions in other subjects:


Mathematics, 05.05.2020 14:31



History, 05.05.2020 14:31

History, 05.05.2020 14:31

History, 05.05.2020 14:31



Mathematics, 05.05.2020 14:31

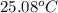 . Or in kelvin temperature will be (25.08 + 273) K = 298.08 K.
. Or in kelvin temperature will be (25.08 + 273) K = 298.08 K.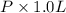 = 0.5 mol \times 0.0821 L atm/mol K \times 298.08 K[/tex]
= 0.5 mol \times 0.0821 L atm/mol K \times 298.08 K[/tex]![[P + a (\frac{n}{V})^{2}] (\frac{V}{n} - b)](/tpl/images/0149/5099/3685f.png) = RT
= RT