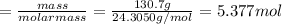Chemistry, 30.07.2019 04:10 luvpeaceandsocc6312
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7 g of magnesium and excess copper(ii) nitrate. mg+cu(no3)2⟶mg(no3)2+cu actual yield:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, akatsionis25
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7...
Questions in other subjects:

Mathematics, 03.06.2020 20:07

Mathematics, 03.06.2020 20:07

Mathematics, 03.06.2020 20:07


Chemistry, 03.06.2020 20:07

Mathematics, 03.06.2020 20:07


Mathematics, 03.06.2020 20:07


Mathematics, 03.06.2020 20:07