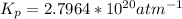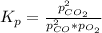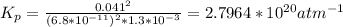Chemistry, 30.07.2019 03:20 nengliangli523
Determine the value of kp for the following reaction if the equilibrium concentrations are as follows: p(co)eq = 6.8 × 10-11 atm, p(o2)eq = 1.3 × 10-3 atm, p(co2)eq = 0.041 atm. 2 co(g) + o2(g) ⇌ 2 co2(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, smm1106
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral. a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Determine the value of kp for the following reaction if the equilibrium concentrations are as follow...
Questions in other subjects:





English, 10.03.2020 04:05