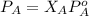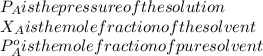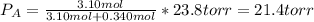Chemistry, 30.07.2019 02:10 keviongardner
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor pressure ph2o of the resulting solution? the vapor pressure of pure water is 23.8 torr at 25 ∘c . express your answer with the appropriate units.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor p...
Questions in other subjects:

Mathematics, 17.01.2021 19:50


Mathematics, 17.01.2021 19:50

Mathematics, 17.01.2021 19:50


Mathematics, 17.01.2021 19:50

Social Studies, 17.01.2021 19:50

Mathematics, 17.01.2021 19:50

Mathematics, 17.01.2021 19:50

English, 17.01.2021 19:50