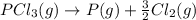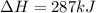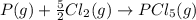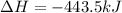Chemistry, 30.07.2019 01:20 jones03riley
Given the enthalpies of reaction: 2p(g) + 3cl2(g) → 2pcl3(g) dh = –574 kj 2p(g) + 5cl2(g) → 2pcl5(g) dh = –887 kj what is the enthalpy change of the following reaction: pcl3(g) + cl2(g) → pcl5(g

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2



Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
Given the enthalpies of reaction: 2p(g) + 3cl2(g) → 2pcl3(g) dh = –574 kj 2p(g) + 5cl2(g) → 2pcl5(g...
Questions in other subjects:

English, 20.09.2019 22:00





Mathematics, 20.09.2019 22:00



Biology, 20.09.2019 22:00

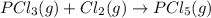
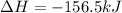
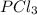 on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.
on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.