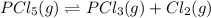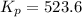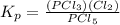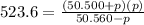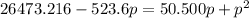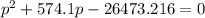Chemistry, 29.07.2019 17:30 davestrider404
For the reaction pcl5(g) 4 pcl3(g) 1 cl2(g) kp 5 23.6 at 500 k a. calculate the equilibrium partial pressures of the reactants and products at 500 k if the initial pressures are ppcl5 5 0.560 atm and ppcl3 5 0.500 atm.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1

Chemistry, 23.06.2019 07:30, mazielynn84
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1
You know the right answer?
For the reaction pcl5(g) 4 pcl3(g) 1 cl2(g) kp 5 23.6 at 500 k a. calculate the equilibrium partial...
Questions in other subjects:

History, 22.01.2022 02:10



English, 22.01.2022 02:10

English, 22.01.2022 02:10


Spanish, 22.01.2022 02:10

English, 22.01.2022 02:10


Mathematics, 22.01.2022 02:10

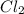 = 42.9 atm,
= 42.9 atm, 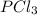 = 93.4 atm and
= 93.4 atm and 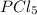 = 7.66 atm
= 7.66 atm