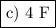G. amount of charge required to reduce
imole of no3-to n2o is
a) 3f
c) 4f
b)...

Chemistry, 28.07.2019 09:10 nicolecadet941
G. amount of charge required to reduce
imole of no3-to n2o is
a) 3f
c) 4f
b) 8f
d) 9f

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1


Chemistry, 23.06.2019 11:20, jaidencoolman7072
When using the ideal gas law constant 0.0821, what unit is used for volume? a) galloonb) ouncec) milliliterd) liter
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 17.07.2020 17:01

Mathematics, 17.07.2020 17:01


Mathematics, 17.07.2020 17:01


Physics, 17.07.2020 17:01

Mathematics, 17.07.2020 17:01