
Chemistry, 27.07.2019 03:20 glowbaby123
A25 -ml of 0.30 m of ammonia, nh (a), solution is titrated with 0.15 m hydrochloric acid, hcl (aq calculate the ph of the solution a) determine the volume of the acid required to reach equivalence point. b) at half the equivalence point. c) at the cquivalence point. d) when 55 ml of the hcl has been added. e choose a suitable indicator for this titration.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
A25 -ml of 0.30 m of ammonia, nh (a), solution is titrated with 0.15 m hydrochloric acid, hcl (aq ca...
Questions in other subjects:

English, 11.03.2021 14:00

English, 11.03.2021 14:00

Health, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00

History, 11.03.2021 14:00




Arts, 11.03.2021 14:00

World Languages, 11.03.2021 14:00

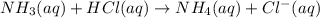
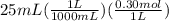
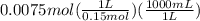
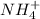 will form and the solution will act as a buffer solution as it has a weak base(ammonia) and its conjugate acid(ammonium ion).
will form and the solution will act as a buffer solution as it has a weak base(ammonia) and its conjugate acid(ammonium ion).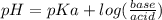
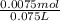
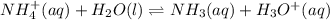
![Ka=\frac{[x][x]}{[0.10-x]}](/tpl/images/0137/3088/1f1fd.png)
![Ka=\frac{[x]^2}{[0.10-x]}Ka for [tex]NH_4^+](/tpl/images/0137/3088/dc53e.png) is
is 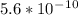 . It's a very low value so the x on the bottom could be neglected and the expression could be written as:
. It's a very low value so the x on the bottom could be neglected and the expression could be written as:
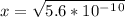
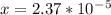
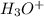 is
is 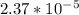 M.
M. 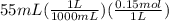
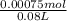 = 0.009375 M
= 0.009375 M

