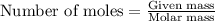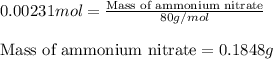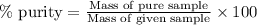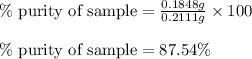Chemistry, 27.07.2019 03:10 JvGaming2001
Ammonium nitrate (nh4n03) is one of the most important nitrogen-containing fertilizers. its purity can be analyzed by titrating a solution of nh4no3 with a standard naoh solution. in one experiment a 0.2111-g sample of industrially prepared nh4no3 required 22.62 ml of 0.1023 m naoh for neutralization (a) enter a net ionic equation for the reaction (include states of matter) (b) what is the percent purity of the sample?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
Ammonium nitrate (nh4n03) is one of the most important nitrogen-containing fertilizers. its purity c...
Questions in other subjects:


Mathematics, 09.03.2021 19:00



Mathematics, 09.03.2021 19:00

Biology, 09.03.2021 19:00

Geography, 09.03.2021 19:00



Chemistry, 09.03.2021 19:00

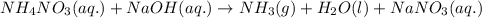
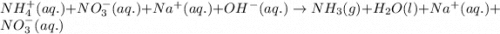
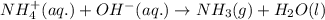
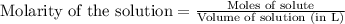
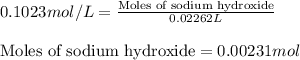
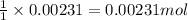 of ammonium nitrate.
of ammonium nitrate.