
Chemistry, 27.07.2019 02:30 Rockey3876
How many grams of ice would be melted by the energy obtained as 16.1 g of steam is condensed at 100°c and cooled to 0°c? specific heat (ice) = 2.10 j/gºc specific heat (water) = 4.18 j/gºc heat of fusion = 333 j/g heat of vaporization = 2258 j/g 43.1 kg 36.4 kg 129 g 6.73 kg 20 g

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
How many grams of ice would be melted by the energy obtained as 16.1 g of steam is condensed at 100°...
Questions in other subjects:



Mathematics, 29.03.2021 21:00


Geography, 29.03.2021 21:00


History, 29.03.2021 21:00


Biology, 29.03.2021 21:00

Computers and Technology, 29.03.2021 21:00

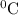
![[16.1\times 2258]J+[16.1\times 4.18\times (100-0)]J](/tpl/images/0137/1545/534f5.png) = 43083.6 J
= 43083.6 J

