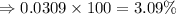Chemistry, 27.07.2019 01:20 ValeryGi3721
"a sample of silicon has an average atomic mass of 28.084amu. in the sample, there are three isotopic forms of silicon. about 92.22% of silicon atoms are 27.9769amu, which have the mass of 28si; about 4.68% are 28.9764amu, which have the mass of 29si, and the remaining isotope, 30si, has a mass of 29.9737amu. calculate the percent isotopic composition of 30si."

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, gizmo50245
Calculate the mass percent of hydrogen in methyl acetate
Answers: 1

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
"a sample of silicon has an average atomic mass of 28.084amu. in the sample, there are three isotopi...
Questions in other subjects:


Mathematics, 03.01.2021 19:10

Physics, 03.01.2021 19:10

Mathematics, 03.01.2021 19:10

Social Studies, 03.01.2021 19:10

History, 03.01.2021 19:10


English, 03.01.2021 19:10

Social Studies, 03.01.2021 19:10

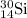 isotope is 3.09 %.
isotope is 3.09 %. .....(1)
.....(1)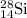 isotope be 'x'
isotope be 'x' isotope:
isotope:![28.084=[(27.9769\times 0.9222)+(28.9764\times 0.0468)+(29.9737\times x)]\\\\x=0.0309](/tpl/images/0136/9885/23b7c.png)