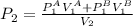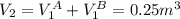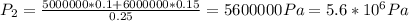Chemistry, 26.07.2019 20:30 nickname278
Atank has a volume of 0.1 m3 and is filled with he gas at a pressure of 5 x 106 pa. a second tank has a volume of 0.15 m' and is filled with he gas at a pressure of 6 x 106 pa. a valve connecting the two tanks is opened. assumıng he to be a monatomic ideal gas and the walls of the tanks to be adiabatic and rigıd, find the final pressure of the system hint: note that the internal energy is constant.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Atank has a volume of 0.1 m3 and is filled with he gas at a pressure of 5 x 106 pa. a second tank ha...
Questions in other subjects:




Medicine, 18.06.2021 16:20



History, 18.06.2021 16:20



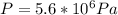
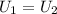 where U1 and U2 are the internal energies before the process and after that respectively.
where U1 and U2 are the internal energies before the process and after that respectively.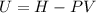 , and that the internal energy of the first state is the sum of the internal energy of each tank.
, and that the internal energy of the first state is the sum of the internal energy of each tank.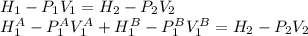
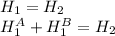
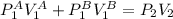
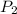 ,
,