
Chemistry, 26.07.2019 20:30 joeblaszak4776
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heated at a constant pressure of 0.5 bar from 100 °c to such a temperature that the specific volume increases by 2.5 times. if the amount of heat that must be added to accomplish this change is 500 kj, calculate the final temperature of the steam, the expansion work, and the change in internal energy.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, 1020lakyn
Subduction zones occur on earth where dense oceanic crust dives under more buoyant continental crust. these boundaries are characterized by a deep ocean trench next to a high continental mountain range, large numbers of earthquakes and volcanoes. all of this is further evidence for the a) big bang theory b) origin of the species eliminate c theory of plate tectonics d theory of natural selection 4 sedimentary rocks found high in the himalayen mountain
Answers: 1

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 02:30, paulinahunl17
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heate...
Questions in other subjects:

Mathematics, 12.09.2021 15:40

English, 12.09.2021 15:40





Biology, 12.09.2021 15:40

Chemistry, 12.09.2021 15:40

Chemistry, 12.09.2021 15:40

Social Studies, 12.09.2021 15:40

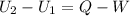
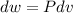
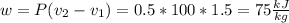 (There is a multiplication by 100 due to the conversion of bar to kPa)
(There is a multiplication by 100 due to the conversion of bar to kPa)
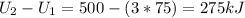
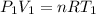
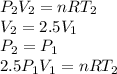
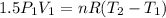
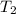 :
: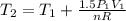
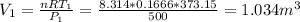
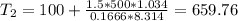 ºC
ºC

