Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
Use tabulated standard electrode potentials to calculate the standard cell potential for the followi...
Questions in other subjects:


Spanish, 04.05.2020 23:59


Mathematics, 04.05.2020 23:59






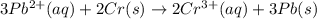
![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0133/7776/82211.png)
![E^0_{[Cr^{3+}/Cr]}=1.33V](/tpl/images/0133/7776/e4046.png)
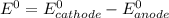
![E^0=E^0_{[Pb^{2+}/Pb]}-E^0_{[Cr^{3+}/Cr]}](/tpl/images/0133/7776/e8ef0.png)