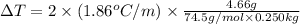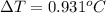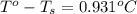Chemistry, 26.07.2019 04:10 zenaidazurita1p6bs1d
Asolution is prepared by dissolving 4.66 g of kcl in enough distilled water to give 250 ml of solution. kcl is a strong electrolyte. how will the freezing point of the solution be different from that of pure water?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2


Chemistry, 23.06.2019 01:00, akluke6059
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 4.66 g of kcl in enough distilled water to give 250 ml of soluti...
Questions in other subjects:


History, 18.06.2021 21:30






Biology, 18.06.2021 21:30


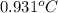 lower than water.
lower than water.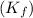 for water =
for water = 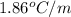
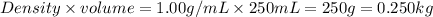
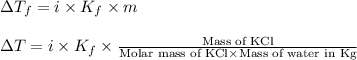
 = change in freezing point
= change in freezing point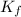 = freezing point constant for water =
= freezing point constant for water =