
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to nitric oxide by the high-temperature reaction 4 nh31g2 + 5 o21g2 ¡ 4 no1g2 + 6 h2o1g2 (a) how is the rate of consumption of o2 related to the rate of consumption of nh3? (b) how are the rates of formation of no and h2o related to the rate of consumption of nh3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3


Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 13:20, brainbean
Use the periodic table to answer the following questions. what is the predicted order of first ionization energies from highest to lowest for beryllium, calcium, magnesium, and strontium? o be > ca > mg > sr o be > mg > ca > sr o ca > sr> be > mg o sr > ca > mg > be done
Answers: 1
You know the right answer?
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to n...
Questions in other subjects:

Social Studies, 24.04.2020 20:11






Mathematics, 24.04.2020 20:11

Mathematics, 24.04.2020 20:11

Mathematics, 24.04.2020 20:11

Mathematics, 24.04.2020 20:11



![rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}=\frac{1}{5}\frac{\Delta [D]}{\Delta t}](/tpl/images/0133/4682/3644a.png)
![rate=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}=-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}](/tpl/images/0133/4682/51a20.png)
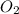 related to rate of consumption of
related to rate of consumption of 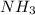 as:
as:![-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/1a857.png)
![\frac{\Delta [O_2]}{\Delta t}=\frac{5}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/ce2db.png)
 the rate of consumption of ammonia.
the rate of consumption of ammonia.![\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/91a55.png)
![\frac{\Delta [NO]}{\Delta t}=-\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/c5068.png)
 to the rate of consumption of ammonia would be:
to the rate of consumption of ammonia would be:![\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/41d66.png)
![\frac{\Delta [H_2O]}{\Delta t}=-\frac{6}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/66644.png)
 times that is 1.5 times to the rate of consumption of ammonia.
times that is 1.5 times to the rate of consumption of ammonia. 

