

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, Britny2386
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
For the reaction below, kp 5 1.16 at 800.8c. caco3(s) 34 cao(s) 1 co2(g) if a 20.0-g sample of caco3...
Questions in other subjects:



History, 29.07.2019 06:40

Mathematics, 29.07.2019 06:40

History, 29.07.2019 06:40


Business, 29.07.2019 06:50



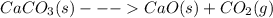
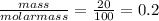
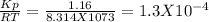
![\frac{[CO_{2}][CaO]}{[CaCO_{3}]}= \frac{x^{2} }{(0.2-x)}=1.3X10^{-4}](/tpl/images/0133/4658/99790.png)
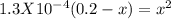
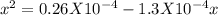
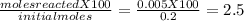 %
%

