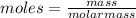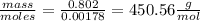Chemistry, 25.07.2019 21:20 Natasha019
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 2.69 °c. the freezing point and kf constant for camphor can be found here. calculate the molar mass of vitamin k.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of...
Questions in other subjects:

Mathematics, 05.07.2019 19:30


Mathematics, 05.07.2019 19:30

History, 05.07.2019 19:30



Mathematics, 05.07.2019 19:30

History, 05.07.2019 19:30