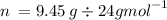Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
Mg metal reacts with hcl to produce hydrogen gas. mg(s)+2hcl(aq)→mgcl2(aq)+h2(g) part a what volume...
Questions in other subjects:

History, 01.09.2019 15:20



Mathematics, 01.09.2019 15:20



Mathematics, 01.09.2019 15:20

Biology, 01.09.2019 15:20

Social Studies, 01.09.2019 15:20