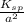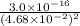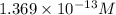Chemistry, 25.07.2019 04:30 wwesuplexcity28
Determine the molar solubility ( ) of zn(cn)2 in a solution with a ph=1.33 . ignore activities. the for zn(cn)2 is 3.0×10−16 . the for hcn is 6.2×10−10 .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Determine the molar solubility ( ) of zn(cn)2 in a solution with a ph=1.33 . ignore activities. the...
Questions in other subjects:


Mathematics, 07.04.2020 02:46

Mathematics, 07.04.2020 02:46

English, 07.04.2020 02:46


History, 07.04.2020 02:46

Mathematics, 07.04.2020 02:46


Mathematics, 07.04.2020 02:46

Mathematics, 07.04.2020 02:47

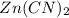 in the given solution.
in the given solution.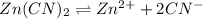
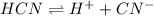
![[H^{+}]](/tpl/images/0129/7944/85507.png)
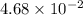
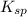 =
= ![[Zn^{2+}][CN^{-}]^{2}](/tpl/images/0129/7944/9ff82.png)
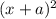
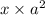 ( x + a = a because a >> x)
( x + a = a because a >> x)