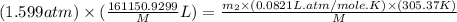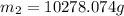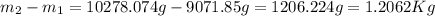Chemistry, 25.07.2019 03:30 cristinaarmijo1
Arigid tank contains 20 lbm of air at 20 psia and 70°f. more air is added to the tank until the pressure and temperature rise to 23.5 psia and 90°f, respectively. determine the amount of air added to the tank. the gas constant of air is r

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, grayfaith16
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Arigid tank contains 20 lbm of air at 20 psia and 70°f. more air is added to the tank until the pres...
Questions in other subjects:


Computers and Technology, 04.10.2020 16:01






Social Studies, 04.10.2020 16:01


Mathematics, 04.10.2020 16:01

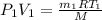
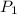 = initial pressure of air =
= initial pressure of air = 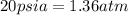
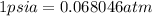
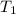 = initial temperature of air =
= initial temperature of air = 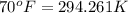
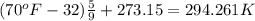
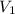 = initial volume of air = ?
= initial volume of air = ?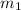 = initial mass of air =
= initial mass of air = 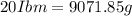
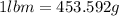
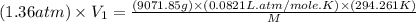
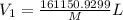
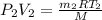
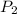 = final pressure of air =
= final pressure of air = 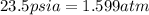
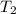 = final temperature of air =
= final temperature of air = 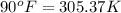
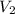 = final volume of air =
= final volume of air = 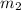 = final mass of air = ?
= final mass of air = ?