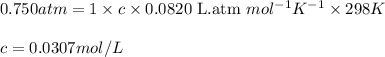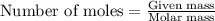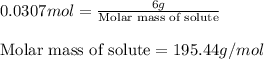Chemistry, 25.07.2019 03:10 alexiaalfaro
Asolution is prepared by dissolving 6.00 g of an unknown nonelectrolyte in enough water to make 1.00 l of solution. the osmotic pressure of this solution is 0.750 atm at 25.0°c. what is the molecular weight (g/mol) of the unknown solute? g

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 6.00 g of an unknown nonelectrolyte in enough water to make 1.00...
Questions in other subjects:



Mathematics, 03.06.2020 03:00



Mathematics, 03.06.2020 03:00

History, 03.06.2020 03:00



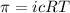
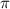 = osmotic pressure of the solution = 0.750 atm
= osmotic pressure of the solution = 0.750 atm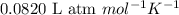
![25^oC=[273+25]=298K](/tpl/images/0129/6185/6a9f9.png)