

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 07:00, jennnifercrd59jc
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2
You know the right answer?
Hemoglobin, a protein in red blood cells, carries o2 from the lungs to the body's cells. iron (as fe...
Questions in other subjects:

History, 27.06.2020 05:01

Spanish, 27.06.2020 05:01

Chemistry, 27.06.2020 05:01

History, 27.06.2020 05:01

History, 27.06.2020 05:01

Mathematics, 27.06.2020 05:01

Law, 27.06.2020 05:01

History, 27.06.2020 05:01


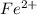 ions in one molecule of hemoglobin are 4.
ions in one molecule of hemoglobin are 4.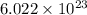 number of atoms.
number of atoms.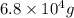
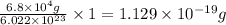
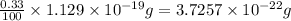
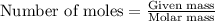

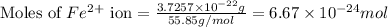
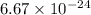 moles of hemoglobin will contain =
moles of hemoglobin will contain = 


