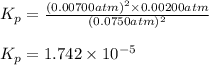Chemistry, 25.07.2019 01:20 jaymee2904p88tgh
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial pressures of h2o, h2, and o2 reach 0.0750 atm, 0.00700 atm, and 0.00200 atm, respectively. what is the value of the equilibrium constant at this temperature?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1


Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial...
Questions in other subjects:


Business, 13.02.2022 08:30


Mathematics, 13.02.2022 08:30

Chemistry, 13.02.2022 08:30

Mathematics, 13.02.2022 08:30


Mathematics, 13.02.2022 08:30

Mathematics, 13.02.2022 08:30

 is the value of the equilibrium constant at this temperature.
is the value of the equilibrium constant at this temperature.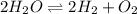


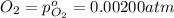
 for the given chemical equation is:
for the given chemical equation is: