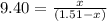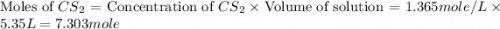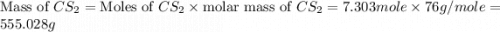Chemistry, 24.07.2019 22:20 nikejose11
Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀cs2(=9.40 at 900 k how many grams of cs2(g) can be prepared by heating 8.08 mol s2(g) with excess carbon in a 5.35 l reaction vessel held at 900 k until equilibrium is attained?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 04:00, zakarycrane8101
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 05:00, andrwisawesome0
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀...
Questions in other subjects:


Mathematics, 08.01.2021 03:30




Geography, 08.01.2021 03:30


Business, 08.01.2021 03:30


Mathematics, 08.01.2021 03:30

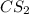 is, 555.028 grams
is, 555.028 grams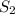 .
.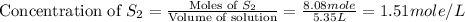
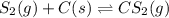
![K_c=\frac{[CS_2]}{[S_2]}](/tpl/images/0128/8287/0a94f.png)