
Chemistry, 24.07.2019 20:10 juandavidklingera553
Consider the combination reaction of samarium metal and oxygen gas. if you start with 33.7 moles of samarium metal, how many moles of oxygen gas would be required to react completely with all of the samarium metal? for this reaction, samarium has a +3 oxidation state within the samarium/oxygen compound.

Answers: 3


Other questions on the subject: Chemistry

You know the right answer?
Consider the combination reaction of samarium metal and oxygen gas. if you start with 33.7 moles of...
Questions in other subjects:

English, 14.01.2020 02:31

Biology, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31

Spanish, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31

History, 14.01.2020 02:31

English, 14.01.2020 02:31


Mathematics, 14.01.2020 02:31

Social Studies, 14.01.2020 02:31

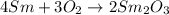
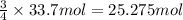 of oxygen gas.
of oxygen gas.

