Consider the half reactions below for a chemical reaction.
mg— mg2(aq) + 2e-
2h* (aq) + 2e...

Consider the half reactions below for a chemical reaction.
mg— mg2(aq) + 2e-
2h* (aq) + 2e--> h2(g)
what is the overall equation for this chemical reaction?
mg(s)+2h+ (aq) — mg2+ (aq) + h2(
mg(s) + 2h+ (aq) —> mg2+ (aq) + 2e-
mg2+ (aq)+h2(9)—> mg(s)+ 2h+ (aq)
mg(s)+2h--> mg(aq) + h2(g)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1


Chemistry, 23.06.2019 00:30, StayPuftMarshadowMan
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Questions in other subjects:










 → Mg²⁺
→ Mg²⁺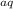 + 2e⁻
+ 2e⁻



