
You have two 466.0 ml aqueous solutions. solution a is a solution of silver nitrate, and solution b is a solution of potassium chromate. the masses of the solutes in each of the solutions are the same. when the solutions are added together, a blood-red precipitate forms. after the reaction has gone to completion, you dry the solid and find that it has a mass of 331.8 g. (a) calculate the concentration of the potassium ions in the original potassium chromate solution.(b) calculate the concentration of the chromate ions in the final solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
You have two 466.0 ml aqueous solutions. solution a is a solution of silver nitrate, and solution b...
Questions in other subjects:




Spanish, 16.04.2021 04:20

Health, 16.04.2021 04:20

Mathematics, 16.04.2021 04:20

English, 16.04.2021 04:20

Chemistry, 16.04.2021 04:20


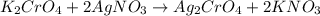
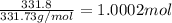
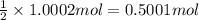 of silver nitrate.
of silver nitrate.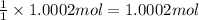 of potassium chromate
of potassium chromate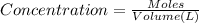
![[K^+]=\frac{2.0004 mol}{0.466 L}=4.2927 mol/L](/tpl/images/0127/0717/630d0.png)
![[CrO_4^{2+}]=\frac{1.0002 mol}{0.466 L+0.466L}=1.0731 mol/L](/tpl/images/0127/0717/e3c96.png)


