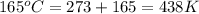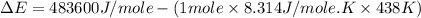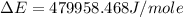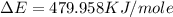Chemistry, 24.07.2019 11:20 brookeanne723
Consider the reaction 2h2o(g) → 2h2(g) + o2(g)δh = 483.6 kj/mol. if 2.0 moles of h2o(g) are converted to h2(g) and o2(g) against a pressure of 1.0 atm at 165°c, what is δu for this reaction?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

You know the right answer?
Consider the reaction 2h2o(g) → 2h2(g) + o2(g)δh = 483.6 kj/mol. if 2.0 moles of h2o(g) are converte...
Questions in other subjects:




Mathematics, 11.12.2021 22:00




Chemistry, 11.12.2021 22:10

English, 11.12.2021 22:10

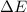 of the reaction is, 479.958 KJ/mole
of the reaction is, 479.958 KJ/mole
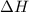 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J/mole
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J/mole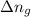 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole