
Chemistry, 23.07.2019 06:20 rainbowboy9231
Agaseous compound is 78.14 percent boron and 21.86 percent hydrogen. at 27°c, 74.3 ml of the gas exerted a pressure of 1.12 atm. if the mass of the gas was 0.0934 g, what is its molecular formula?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

You know the right answer?
Agaseous compound is 78.14 percent boron and 21.86 percent hydrogen. at 27°c, 74.3 ml of the gas exe...
Questions in other subjects:





Mathematics, 09.02.2022 01:30



Mathematics, 09.02.2022 01:30

Mathematics, 09.02.2022 01:30

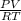
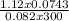
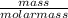
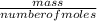
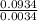
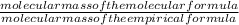
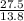 = 2
= 2

