
Atmospheric pressure at sea level is 760 mm hg, and oxygen makes up 20.9% of this air when it is dry. scientists at the mt. washington observatory in new hampshire measured the atmospheric pressure at the summit of mt. washington (6,289 feet above sea level) as 609 mm hg. when the air is dry, the partial pressure of oxygen at the summit is approximately mm hg.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

You know the right answer?
Atmospheric pressure at sea level is 760 mm hg, and oxygen makes up 20.9% of this air when it is dry...
Questions in other subjects:

English, 09.02.2021 02:30


Chemistry, 09.02.2021 02:30



Engineering, 09.02.2021 02:30



English, 09.02.2021 02:30

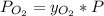
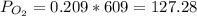 mmHg.
mmHg.


