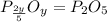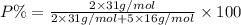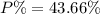Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3


Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
When a 35.07 g sample of phosphorus reacts with oxygen a 71.00 g sample of phosphorus oxide is forme...
Questions in other subjects:

English, 02.03.2020 23:29






Mathematics, 02.03.2020 23:29



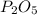 is the empirical formula for this compound.
is the empirical formula for this compound.

 moles of phosphorus oxide...(1)
moles of phosphorus oxide...(1)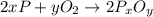
 moles of phosphorus oxide...(2)
moles of phosphorus oxide...(2)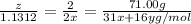
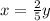
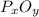 :
: