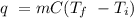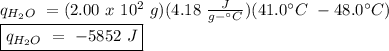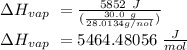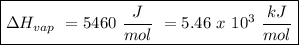Chemistry, 23.07.2019 02:20 bryanmcmillianjr
The heat of vaporization of a liquid (δhvap) is the energy required to vaporize 1.00 g of the liquid at its boiling point. in one experiment, 30.0 g of liquid nitrogen (boiling point = −196°c) is poured into a styrofoam cup containing 2.00 × 102 g of water at 48.1°c. calculate the molar heat of vaporization of liquid nitrogen if the final temperature of the water is 41.0°c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
The heat of vaporization of a liquid (δhvap) is the energy required to vaporize 1.00 g of the liquid...
Questions in other subjects:

Mathematics, 22.02.2021 21:20

Chemistry, 22.02.2021 21:20

Mathematics, 22.02.2021 21:20

Chemistry, 22.02.2021 21:20


Mathematics, 22.02.2021 21:20

Mathematics, 22.02.2021 21:20

Physics, 22.02.2021 21:20

History, 22.02.2021 21:20

Spanish, 22.02.2021 21:20