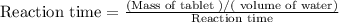Assume that each tablet's mass was 1,000 mg, and remember that you used 0,200 l of water each time,
compute the reaction rate to the nearest whole number using the formula below,
mass of tablet/volume of water
reaction rate = mas
reaction time
3°c reaction time = 138.5 sec
reaction rate = mg/l/sec
24°c reaction time = 34,2 sec
reaction rate = mg/l/sec
40°c reaction time = 26.3 sec
reaction rate = mg/l/sec
65°c reaction time = 14.2 sec
reaction rate = mg/l/sec


Answers: 3


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 07:30, Ayyyyeeeeeeewuzgud
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
Assume that each tablet's mass was 1,000 mg, and remember that you used 0,200 l of water each time,<...
Questions in other subjects:



Mathematics, 23.06.2019 10:30



History, 23.06.2019 10:30

Spanish, 23.06.2019 10:30

Mathematics, 23.06.2019 10:30


 is, 36.1 mg/L/sec
is, 36.1 mg/L/sec is, 146.2 mg/L/sec
is, 146.2 mg/L/sec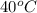 is, 190.1 mg/L/sec
is, 190.1 mg/L/sec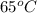 is, 352.1 mg/L/sec
is, 352.1 mg/L/sec