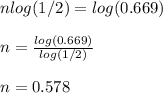Chemistry, 22.07.2019 18:10 sammyraegarrett
The carbon−14 decay rate of a sample obtained from a young tree is 0.266 disintegration per second per gram of the sample. another wood sample prepared from an object recovered at an archaeological excavation gives a decay rate of 0.178 disintegration per second per gram of the sample. what is the age of the object? (the half-life of carbon−14 is 5715 years.) × 10 years enter your answer in scientific notation.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2


Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
The carbon−14 decay rate of a sample obtained from a young tree is 0.266 disintegration per second p...
Questions in other subjects:

Chemistry, 10.10.2021 17:50

Mathematics, 10.10.2021 17:50

Mathematics, 10.10.2021 17:50


Spanish, 10.10.2021 17:50





Business, 10.10.2021 17:50