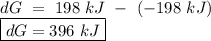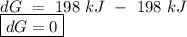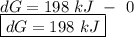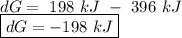Chemistry, 21.07.2019 01:10 savyblue1724707
1point
for a reaction, a h= 198 kj. for which value of ta sis the reaction
spontaneous?
o a. -198 kj
o b. 198 kj
o c. oku
o d. 396 kj
submit

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fespinoza019
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
1point
for a reaction, a h= 198 kj. for which value of ta sis the reaction
spontaneous?...
for a reaction, a h= 198 kj. for which value of ta sis the reaction
spontaneous?...
Questions in other subjects:

Biology, 06.05.2020 01:24

Social Studies, 06.05.2020 01:24


History, 06.05.2020 01:24


Mathematics, 06.05.2020 01:24


Biology, 06.05.2020 01:24

Biology, 06.05.2020 01:24

Mathematics, 06.05.2020 01:24

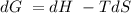 A positive ΔG represents a non-spontaneous reactionA negative ΔG value indicates a spontaneous reaction.
A positive ΔG represents a non-spontaneous reactionA negative ΔG value indicates a spontaneous reaction.