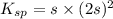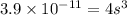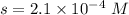What is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant for caf2 is 3.9 ⋅ 10-11 at 25 °c. what is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant for caf2 is 3.9 10-11 at 25 °c. 3.4 ⋅ 10-4 8.8 ⋅ 10-6 3.1 ⋅ 10-6 1.3 ⋅ 10-11 2.1 ⋅ 10-4

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

You know the right answer?
What is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant...
Questions in other subjects:

Social Studies, 23.01.2021 14:20


English, 23.01.2021 14:20



Social Studies, 23.01.2021 14:20

Business, 23.01.2021 14:20


Mathematics, 23.01.2021 14:20

Chemistry, 23.01.2021 14:20

![K_{sp}=\left[Ca^{2+} \right]\left[F^{-} \right]^2](/tpl/images/0110/5296/1ebe5.png)