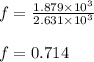Chemistry, 20.07.2019 02:30 aubriebv2020
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) how many moles of oxygen are present? (take the molar mass of oxygen to be 32.0 g/mol) (b) how much energy is transferred to the oxygen as heat? (the molecules rotate but do not oscillate.) (c) what fraction of the heat is used to raise the internal energy of the oxygen?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2

Chemistry, 23.06.2019 03:00, KindaSmartPersonn
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) h...
Questions in other subjects:

History, 25.01.2021 16:20

English, 25.01.2021 16:20

Business, 25.01.2021 16:20


Mathematics, 25.01.2021 16:20


Mathematics, 25.01.2021 16:20

Mathematics, 25.01.2021 16:20


History, 25.01.2021 16:20





 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure =  (For diatomic gas)
(For diatomic gas) = change in temperature =
= change in temperature = 

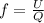

 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure =  (For diatomic gas)
(For diatomic gas)