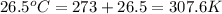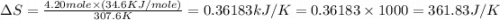Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1


Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Calculate the change in entropy that occurs in the system when 4.20 mole of diethyl ether (\(\rm c_4...
Questions in other subjects:

Mathematics, 28.07.2019 09:30

Mathematics, 28.07.2019 09:30


Mathematics, 28.07.2019 09:30

Spanish, 28.07.2019 09:30





Mathematics, 28.07.2019 09:30

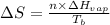
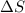 = entropy change of the system = ?
= entropy change of the system = ?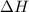 = enthalpy of vaporization = 34.6 kJ/mole
= enthalpy of vaporization = 34.6 kJ/mole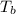 = normal boiling point =
= normal boiling point =