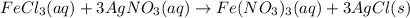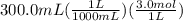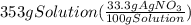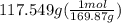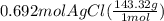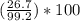Chemistry, 19.07.2019 20:10 laurielaparr2930
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass % solution of silver (u) nitrate and water. 267 grams of a solid precipitate forms. what is the percent yield of the reaction assuming that the solubility of the solid precipitate in water is negligible.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass %...
Questions in other subjects:

Mathematics, 08.10.2020 01:01


Physics, 08.10.2020 01:01


History, 08.10.2020 01:01

Mathematics, 08.10.2020 01:01

English, 08.10.2020 01:01



Geography, 08.10.2020 01:01