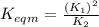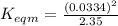Chemistry, 19.07.2019 04:30 yarielisr18
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the reaction 2a(s)⇌3d(g). a(s) ⇌ 12 b(g)+c(g), k1=0.0334 3d(g) ⇌ b(g)+2c(g), k2=2.35

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the...
Questions in other subjects:

English, 03.09.2021 14:00

Medicine, 03.09.2021 14:00

Spanish, 03.09.2021 14:00




Health, 03.09.2021 14:00

Biology, 03.09.2021 14:00

Mathematics, 03.09.2021 14:00

English, 03.09.2021 14:00


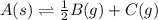
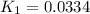
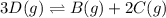



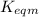 for the final reaction.
for the final reaction.