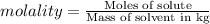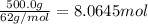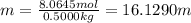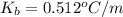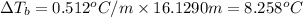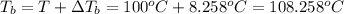Chemistry, 19.07.2019 04:10 zlittleton2008
Calculate the boiling point of a solution of 500.0 g of ethylene glycol (c2h6o2) dissolved in 500.0 g of water. kf = 1.86°c/m and kb = 0.512°c/m. use 100°c as the boiling point of water.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 01:30, Slycooper5959
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1
You know the right answer?
Calculate the boiling point of a solution of 500.0 g of ethylene glycol (c2h6o2) dissolved in 500.0...
Questions in other subjects:




Geography, 20.11.2020 18:40



Mathematics, 20.11.2020 18:40

English, 20.11.2020 18:40


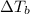
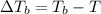
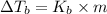
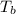 = Boiling point of the solution
= Boiling point of the solution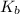 = Molal elevation constant of solvent
= Molal elevation constant of solvent