
Chemistry, 19.07.2019 03:30 Wolfgirl2032
Which of the following assumptions appears reasonable for the isothermal process? h20 (liq, 1 bar) → h2o (liq, 1300 bar), t = 20°c. a. au = 0, ah 0 b. au = 0, ah 0 c. au #0, ah = 0 d. none of the above hinn nf diethyl ether using the chen's rule is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Which of the following assumptions appears reasonable for the isothermal process? h20 (liq, 1 bar)...
Questions in other subjects:


English, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00

History, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

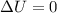 and
and 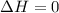


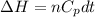
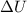 = internal energy
= internal energy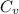 = specific heat capacity at constant volume
= specific heat capacity at constant volume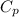 = specific heat capacity at constant pressure
= specific heat capacity at constant pressure = change in temperature
= change in temperature = 0
= 0

