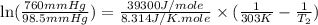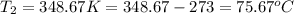Chemistry, 19.07.2019 00:10 melanie7152
The vapor pressure of ethanol is 30°c at 98.5 mmhg and the heat of vaporization is 39.3 kj/mol. determine the normal boiling point of ethanol from this data.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
The vapor pressure of ethanol is 30°c at 98.5 mmhg and the heat of vaporization is 39.3 kj/mol. dete...
Questions in other subjects:

Mathematics, 16.12.2019 00:31


Mathematics, 16.12.2019 00:31

English, 16.12.2019 00:31



Biology, 16.12.2019 00:31

Mathematics, 16.12.2019 00:31


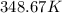 or
or 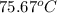
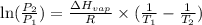
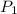 = vapor pressure of ethanol at
= vapor pressure of ethanol at 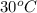 = 98.5 mmHg
= 98.5 mmHg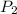 = vapor pressure of ethanol at normal boiling point = 1 atm = 760 mmHg
= vapor pressure of ethanol at normal boiling point = 1 atm = 760 mmHg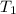 = temperature of ethanol =
= temperature of ethanol = 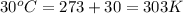
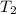 = normal boiling point of ethanol = ?
= normal boiling point of ethanol = ?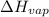 = heat of vaporization = 39.3 kJ/mole = 39300 J/mole
= heat of vaporization = 39.3 kJ/mole = 39300 J/mole