

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium...
Questions in other subjects:

Mathematics, 17.04.2021 14:00


History, 17.04.2021 14:00

Geography, 17.04.2021 14:00

Geography, 17.04.2021 14:00

Computers and Technology, 17.04.2021 14:00

English, 17.04.2021 14:00

Computers and Technology, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00

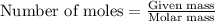 ....(1)
....(1)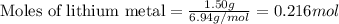
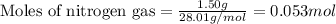
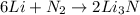
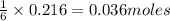 of nitrogen gas.
of nitrogen gas.

