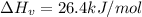Chemistry, 18.07.2019 22:30 crybaby222psyc
The estimated heat of vaporization of diethyl ether using the chen's rule is a. 29.7 kj/mol b. 33.5 kj/mol c. 26.4 kj/mol d. 36.8 kj/mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2



Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
The estimated heat of vaporization of diethyl ether using the chen's rule is a. 29.7 kj/mol b. 33.5...
Questions in other subjects:


Mathematics, 13.06.2020 17:57

Mathematics, 13.06.2020 17:57


History, 13.06.2020 17:57

English, 13.06.2020 17:57

Mathematics, 13.06.2020 17:57

Mathematics, 13.06.2020 17:57

Mathematics, 13.06.2020 17:57

History, 13.06.2020 17:57

![\Delta H_v=RT_b\left [ \frac{3.974\left ( \frac{T_b}{T_c} \right )-3.958+1.555lnP_c}{1.07-\left ( \frac{T_b}{T_c} \right )} \right ]](/tpl/images/0105/5856/32a5b.png)
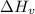 is the Heat of vaoprization (J/mol)
is the Heat of vaoprization (J/mol)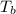 is the normal boiling point of the gas (K)
is the normal boiling point of the gas (K) is the Critical temperature of the gas (K)
is the Critical temperature of the gas (K)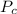 is the Critical pressure of the gas (bar)
is the Critical pressure of the gas (bar)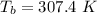
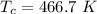
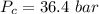
![\Delta H_v=8.314\times307.4 \left [ \frac{3.974\left ( \frac{307.4}{466.7} \right )-3.958+1.555ln36.4}{1.07-\left ( \frac{307.4}{466.7} \right )} \right ]](/tpl/images/0105/5856/04a03.png)