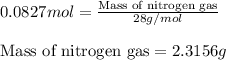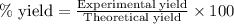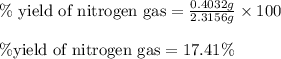Chemistry, 18.07.2019 22:10 jgstyle2388
Hydrazine, n2h4 , reacts with oxygen to form nitrogen gas and water. n2h4(aq)+o2(g)⟶n2(g)+2h2o(l) if 2.65 g of n2h4 reacts with excess oxygen and produces 0.350 l of n2 , at 295 k and 1.00 atm, what is the percent yield of the reaction?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Hydrazine, n2h4 , reacts with oxygen to form nitrogen gas and water. n2h4(aq)+o2(g)⟶n2(g)+2h2o(l) if...
Questions in other subjects:

Mathematics, 03.06.2021 16:50

Chemistry, 03.06.2021 16:50



Mathematics, 03.06.2021 16:50

English, 03.06.2021 16:50

Spanish, 03.06.2021 16:50


English, 03.06.2021 16:50

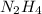
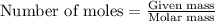
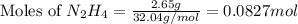
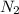
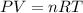
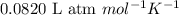
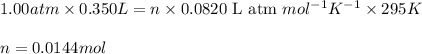
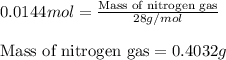
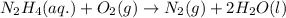
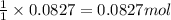 of nitrogen gas.
of nitrogen gas.